
Welcome to this video tutorial on IV fluids. IV fluids can be placed in two general categories: colloids and crystalloids. Our focus for this lesson will be crystalloids, which is a watery-type solution of mineral salts and other water-soluble molecules.
Crystalloid solutions contain small molecules that flow easily through semipermeable membranes, allowing for transfer from the bloodstream into the cells and body tissues. Crystalloid solutions are the most commonly used IV solutions for fluid replacement.
There are three types of crystalloid solutions: isotonic solutions, hypotonic solutions, and hypertonic solutions.
Isotonic Solutions
In an isotonic solution, the osmotic pressure is constant inside and outside of the cells. No shift of fluid occurs and the cells don’t shrink or swell.
They are given to replace extracellular fluid losses and to expand intravascular volume. Some examples of isotopic solutions include normal saline (written as NS, 0.9% sodium chloride), lactate ringer (written as LR), and 5% dextrose in water (written as D5W).
Normal Saline
Normal saline is simply saltwater. It contains water, sodium, and chloride. The percentage of sodium chloride dissolved in the solution is similar to the usual concentration of sodium in chloride in the intravascular space. Since water follows salt, normal saline increases the fluid volume in extracellular spaces.
Normal saline is the fluid of choice for resuscitation efforts and a fluid challenge. It is also the only solution used when administering blood products.
Lactated Ringer
Lactated ringer has an electrolyte content most closely related to the body’s blood and serum make-up.
Lactated ringer is a good choice for resuscitation efforts and it is beneficial for surgical and burn patients as they benefit from the electrolyte replacement. Lactated ringer contains sodium, chloride, calcium, and potassium.
D5W
D5W is unique. It can be categorized as an isotonic solution or a hypotonic solution. The amount of dextrose in the solution makes its tonicity, or solute concentration, similar to that of intravascular fluid, making it isotonic. It also provides free water, following the metabolism of the dextrose. It is also considered a hypotonic solution.
D5W is basically a sugarwater solution that provides 170 calories per liter but doesn’t provide the electrolyte replacement.
Our next category of IV solutions is hypotonic solutions.
Hypotonic Solutions
Compared with intracellular fluids, hypotonic solutions have a lower concentration of solutes.
Fluid shifts from the intravascular space to both intracellular and interstitial spaces. Simply put, fluid goes into the cell. Remember, water follows salt, so the hypotonic solutions move inside the cell to try to even out the concentration of solutes.
Hypotonic solutions are used to treat intracellular dehydration, such as diabetic ketoacidosis and hypernatremia , which is too much sodium in the blood. The most commonly used hypotonic solution is .45% sodium chloride, usually called half normal saline (written as 1/2 NS, or .45% NS). Also, D5W is hypotonic after metabolism.
Others that are not used as often include .33% sodium chloride. .2% sodium chloride, and 2.5% dextrose in water.
The third type of IV solution is the hypertonic solutions.
Hypertonic Solutions
Compared with intracellular fluids, hypertonic solutions have a higher concentration of solids.
The osmotic pressure draws water out of the cell, increasing extracellular fluid volume. Hypertonic solutions are used as volume expanders. They may be prescribed for patients with severe hyponatremia, which is when the sodium in the blood is too low.
These are some examples of hypertonic solutions: D10W (dextrose 10% in water), D5NS (dextrose 5% in .9% sodium chloride), D5 \(\frac{1}{2}\) NS (dextrose 5% in .45% sodium chloride), and D5LR (dextrose 5% in lactated ringer).
Use caution when administering hypertonic saline solutions because of their potential for causing intravascular fluid volume overload and pulmonary edema. Teach patients to notify a nurse if they develop breathing difficulties or if they feel their heart is beating very fast.
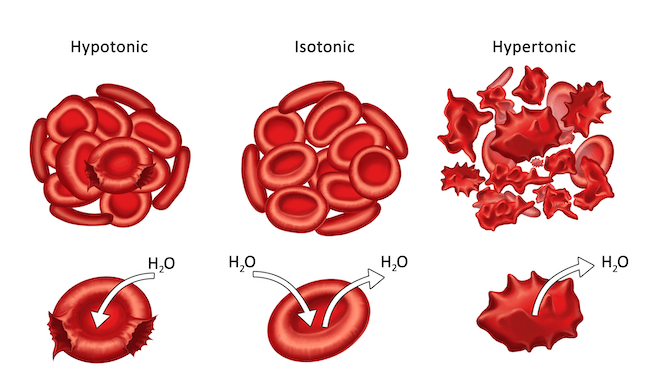
Review
Let’s look at a quick review of the three types of crystalloid IV solutions. Isotonic is when water and solute are at equal levels inside and outside of the cell. There is no shift of fluid in or out of the cell. Hypotonic solutions is when there is less value in the solution and more solute in the cell, so water goes inside the cell. Hypertonic solutions are when more solute is in the solution and less solute in the cell, so water goes outside of the cell.
Thank you for watching this video!
Frequently Asked Questions
Q
What types of IV fluids are used in medical practice?
A
Two commonly administered IV fluids in medical practice are crystalloids and colloids. Crystalloids are by far the most common, as they consist of water and small water-soluble electrolytes that are easily absorbed from the blood stream. They are indicated for fluid maintenance, fluid replacement and resuscitation. Colloids are comprised of larger solutes (most commonly protein) and can be in natural form (blood or plasma) or manufactured form (albumin). Because of the size of the molecules, the fluid is more likely to remain in the intravascular space and for that reason, colloids may be indicated over crystalloids for resuscitation in the context of severe hypovolemia to avoid resulting shock.
Q
What are the three categories of crystalloid IV fluids?
A
Crystalloids are subdivided into three categories based on their tonicity when compared to normal human blood and plasma: isotonic, hypotonic, and hypertonic.
Q
What are isotonic fluids?
A
Isotonic fluids have concentrations similar to human blood and plasma. Their consistent osmotic pressure inside and outside of the cell results in no fluid shift or cell swelling. They are administered to replace fluid loss and expand intravascular volume. Examples include normal saline (0.9% NaCl), Lactated Ringer (LR), and dextrose 5% in water (D5W) prior to the dextrose being metabolized.
Q
What are hypotonic fluids?
A
Hypotonic fluids are those with a lower concentration of solutes than normal human blood and plasma. The osmotic pressure pushes the fluid into the cell from the extracellular space. They are used to treat intracellular dehydration that occurs in conditions such as diabetic ketoacidosis (DKA) and hypernatremia. The most commonly used hypotonic fluid is 45% NaCl. D5W is also considered hypotonic after the dextrose has been metabolized and only free water remains.
Q
What are hypertonic fluids?
A
Hypertonic fluids are those with a higher concentration of solutes than normal human blood and plasma. The osmotic pressure draws fluid out of the cell, increasing extracellular volume in the intravascular space, and therefore acting as a volume expander. They are most often administered to treat hyponatremia, but are contraindicated in congestive heart failure due to the possibility of resulting volume overload or flash pulmonary edema. Examples include D10W, D5NS, and D5LR.
Q
What is normal saline and what is it used for?
A
Normal saline is simply saltwater. It contains water, sodium, and chloride. The percentage of sodium chloride dissolved in the solution is similar to the usual concentration of sodium chloride in the intravascular space. Since water follows salt, normal saline increases the fluid volume in extracellular spaces. Normal saline is the fluid of choice for resuscitation efforts and a fluid challenge. It is also the only solution used when administering blood products.
Q
What is Lactates Ringer solution and what is it used for?
A
Lactated Ringer (LR) has an electrolyte content most closely related to the body’s blood and serum make-up. This makes it a good choice for resuscitation efforts, and it is beneficial for surgical and burn patients as they benefit from the electrolyte replacement. Lactated Ringer contains sodium, chloride, calcium, and potassium.
Q
What is D5W and what is it used for?
A
D5W is unique. It can be categorized as an isotonic solution or a hypotonic solution. The amount of dextrose in the solution makes its tonicity, or solute concentration, similar to that of intravascular fluid, making it isotonic. It also provides free water, following the metabolism of the dextrose. It is also considered a hypotonic solution. D5W is basically a sugar water solution that provides 170 calories per liter but doesn’t provide the electrolyte replacement.

