Hi, and welcome to this video on electron configuration, which is how electrons arrange themselves around an atomic nucleus.
While this may seem a little abstract at first, electron configuration underlies many atomic properties and the layout of the periodic table itself, so it’s pretty important to understand.
First, we’ll start by acquainting ourselves with the electron itself. Once we get to know the electron’s individual characteristics, we’ll have a better foundation for understanding their interactions and configuration around a nucleus. We’ll then introduce atomic orbitals, the regions where electrons live, and go through the notation used to represent them.
Let’s get started!
Electron Properties
An electron is a teeny tiny negatively charged subatomic particle. As far as scientists know, electrons are elementary particles, so you can’t break them down into smaller bits. They are 9.109 x 10-31 kg, which is about 0.0005 times less than the mass of a proton or neutron. Despite being much smaller, the charge of an electron is the exact same magnitude as a proton but of the opposite sign.
Electrons are leptons, a member of the fermion family, which means they have a spin of ½, which we’ll get to in a second, and do not participate in the strong nuclear force. This doesn’t mean they don’t interact strongly with other particles; after all, they’re negatively charged so they’re attracted to positive charges and repulsed by other negative charges.
Let’s return to the spin of 1/2 comment. What does that mean exactly? Spin is an intrinsic characteristic of certain particles that’s, frankly, kind of spooky. As in, scientists don’t have a great explanation for it. We know it exists because the results of certain quantum mechanics experiments demand that it exists, but we can’t explain it with classical physics or in a way that’s intuitive.
In fact, the very name spin is misleading as one would think the electron is spinning on its axis, but that’s not accurate and just leads to misconceptions. This type of thing pops up all the time when you study electrons. We could spend hours discussing the quantum nature of electrons, but that’s beyond this video, and for today, all you’ll need to know is simply that every electron is either “spin up” or “spin down”.
Spin is represented by the spin quantum number s, which can only be 1/2 or -1/2 for an electron and is illustrated with an up or down single-sided arrow.
So far, we’ve learned about the properties of individual electrons—they have mass, charge, and spin. Now, let’s think about how they gather around a nucleus—in other words, their configuration.
Orbitals in Atomic Structure
We start with the electromagnetic force, the negative electron and the positive nucleus are attracted to each other. The electron wants to be close to the nucleus, which maximizes the attraction and results in the most stable configuration. But as more electrons are added to the atom, they must balance the desire to be near the nucleus, while minimizing the repulsion between them.
To achieve the best balance, electrons configure themselves in a very particular way around the nucleus. These three-dimensional regions where electrons live are called orbitals and are precisely calculated using quantum mechanics.
Each atomic orbital is characterized by three quantum numbers, \(n\), \(l\), and \(m\), which describe the size, shape, and spatial orientation of the orbital, respectively. Being a quantum number means that \(n\), \(l\), and \(m\) can only have specific, discrete values.
A full explanation of these quantum numbers is beyond the scope of this video, but we’ll give enough of an introduction for you to understand the notation used to write out electron configurations.
\(n\) is the principal quantum number and is permitted to have positive integer values (1, 2, 3, 4 and so on). As n increases, the energy and size of the orbital increases as well.
\(l\) is the angular momentum quantum number. It describes the shape of the orbital and is permitted to have integer values from 0 to \(n\)-1. For example, if \(n\)=2, \(l\) could be 0 or 1. When \(l\) is 0, the orbital is designated as a sharp, or s, orbital, which is a sphere, and is the standard image most of us have for electron orbitals.
But not all orbitals are spherical. When \(l\) is 1, the orbital is designated as a principal, or p, orbital, which looks like a barbell. As \(l\) increases, the orbital shapes become more complex for the d, diffuse, orbitals when \(l\) equals 2, and f, fundamental, orbitals when \(l\) equals 3.
\(m\) is the magnetic quantum number. It describes the spatial orientation of the orbital and is permitted to have integer values from \(–l\) to \(l\). For example, if \(l\)=2, \(m\) could be -2, -1, 0, 1, or 2.
Putting these quantum numbers together, we get the atomic orbitals.
Now that we’re familiar with the orbitals themselves, we’re almost ready to put some electrons into them! But we’re missing a vital piece of information. How many electrons can exist in each orbital? The answer is two. But why two?
Remember that electrons are fermions. Among other things, this means that no two electrons can have the exact same set of quantum numbers. This is known as the Pauli exclusion principle. Any two electrons in the same orbital would have identical n, l, and m quantum numbers, but their fourth quantum number, their spin, distinguishes them. One electron is spin up (s equals 1/2) and one is spin down (s equals -1/2). Since electrons can only be spin up or spin down, we can’t add any more electrons to the orbital without violating the Pauli exclusion principle.
This means that s orbitals can hold 2 electrons, p orbitals can hold 6 electrons because there are three p orbitals, d orbitals can hold 10 electrons because there are five d orbitals, and f orbitals can hold 14 electrons because there are seven f orbitals.
This actually accounts for the shape of the modern periodic table.
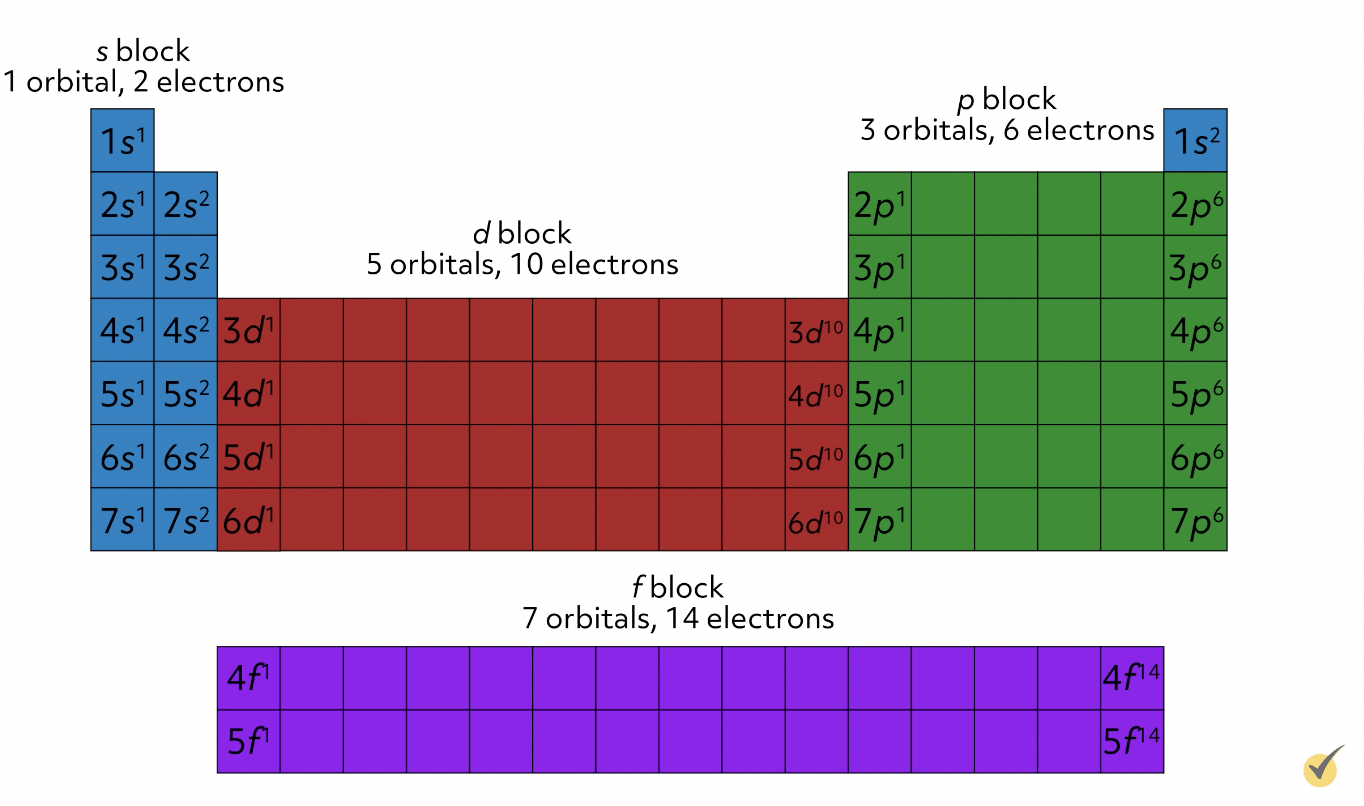
You can split it up into the s block, with two columns, one for each s electron, into the p block with 6 columns, the d block with 10 columns, and the f block with 14 columns. Down each column, the configuration of the valence electrons is the same, which accounts for why elements in the same group have similar properties. For example, in group 17, the halogens, the valence electron configuration of every element is np5, which primarily dictates their characteristics.
Review
Okay, let’s review before we go to make sure you remember everything.
Electrons, which have the same magnitude of charge as a proton and a spin of 1/2, are attracted to nuclei through electromagnetic force. As more electrons are added to an atom, they mostly achieve balance by configuring themselves into orbitals. The shape, size, and spatial organization of these orbitals can be described using \(n\), \(l\), and \(m\) respectively.
There can only be two electrons in a single orbital, one being spin up and the other spin down. And finally, the periodic table can be broken up into four blocks showing the configuration of the valence electrons.
I hope this review was helpful! Thanks for watching, and happy studying!
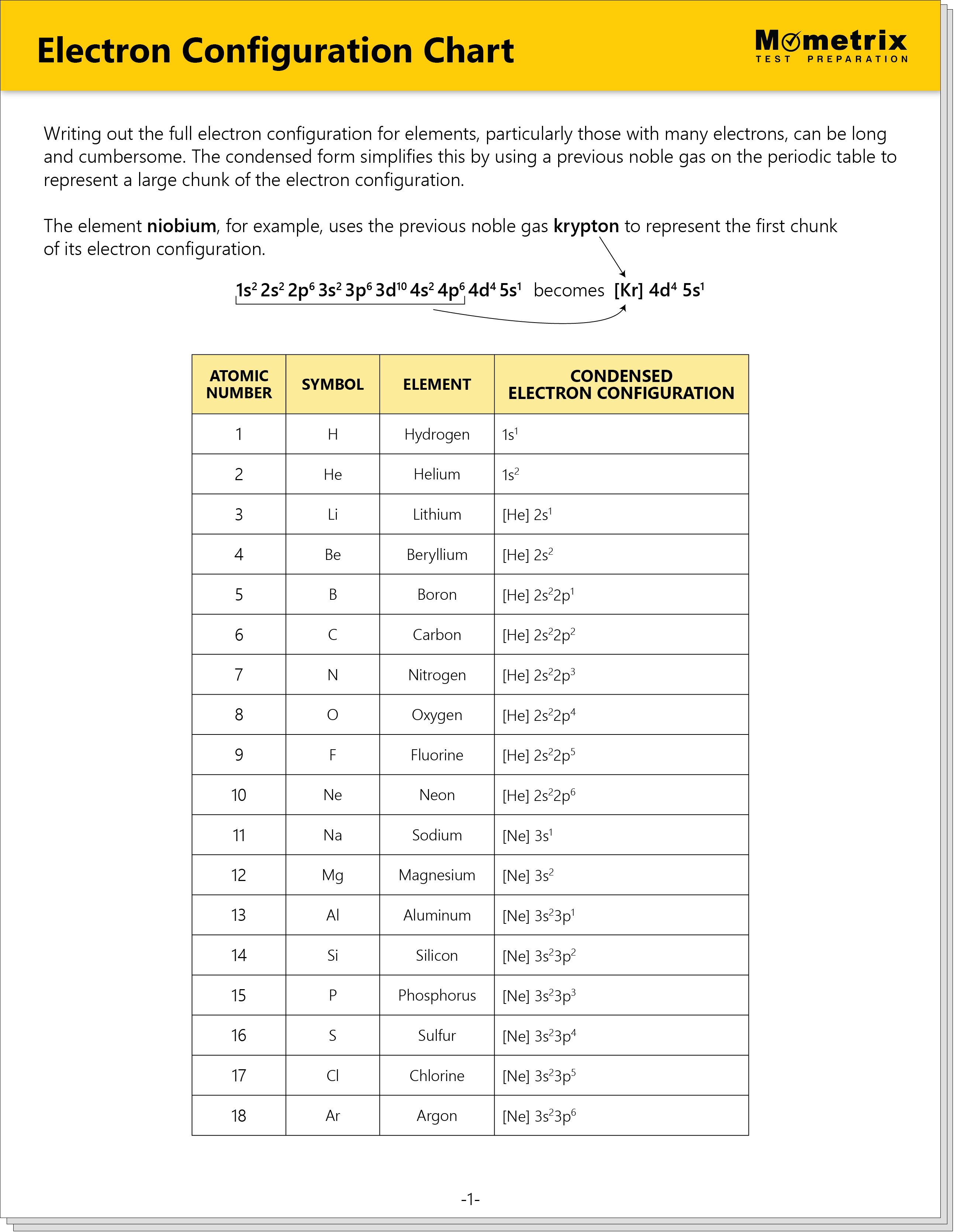
Electron Configuration Chart PDF
Explore our comprehensive Electron Configuration Chart, which includes a copy of the Periodic Table of Elements. This detailed guide simplifies understanding electron configurations of all elements by using the condensed form with noble gas notation. It also outlines three of the most important principles related to electron configuration: the Aufbau Principle, the Pauli Exclusion Principle, and Hund’s First Rule.