
Hi, and welcome to this video on catalysts!
Catalysts come in all shapes and sizes but ultimately, all have the same job: to speed up chemical reactions. To really understand how catalysts work, we need to do a quick review on kinetics. Then, we’ll talk about different types of catalysts and see how they can affect reaction speeds.
Let’s get started!
Chemical Reactions
Let’s start by reviewing chemical reactions. There are two primary things we want to know about any given reaction. First, we want to know the extent of the reaction. In other words, we want to know when the reaction is finished and how much product we will have. This falls under the study of thermodynamics. Second, we want to know how fast the reaction will proceed. This falls under kinetics.
Reaction Coordinate and Activation Energy
Reactions can be incredibly complex, with many transition states and intermediates, but, for now, we’ll focus on a theoretical reaction that’s quite simple.
Note that for this reaction, our reactants are higher in energy than our products. This tells us that the reaction releases energy along the way, which means the change in enthalpy is negative and the reaction is exothermic. This is the thermodynamics of the reaction and directly impacts the equilibrium, in other words, how much reactant will ultimately convert to product.
Now, let’s turn to the kinetics of the reaction. As the reactants convert to products, the energy of the system increases as bonds begin to stretch and break, peaking at the high-energy transition state, before reaching their new low energy configuration. The height of that barrier is the activation energy and determines the rate of the reaction, \(k\).
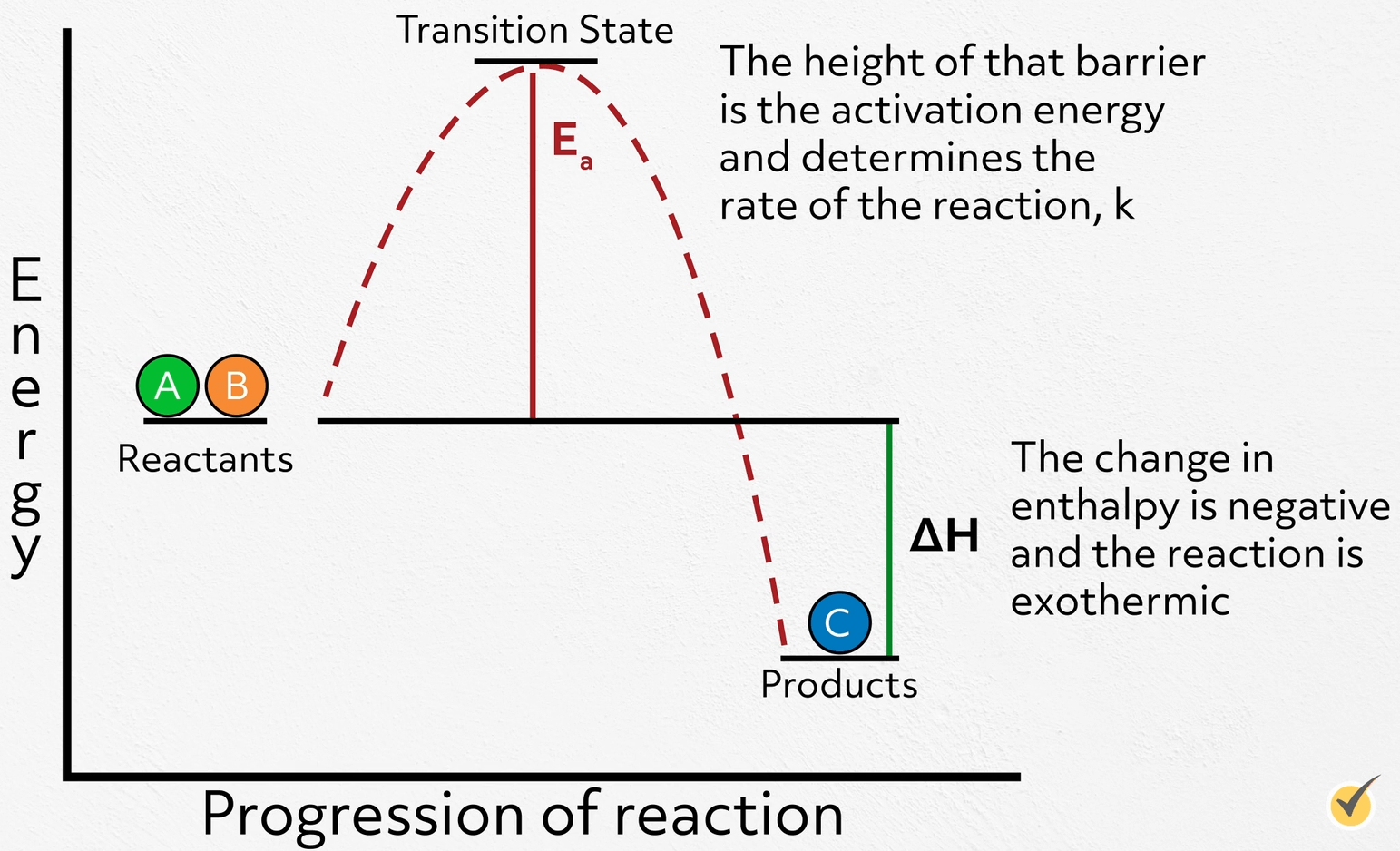
The larger the barrier, the slower the reaction and vice versa.
How Catalysts Work
So where do catalysts fit into all of this? Well, when we talk about speeding up a reaction, we’re talking about changing the activation energy or creating an alternative, lower energy path. And this is exactly what catalysts do!
In our simple reaction coordinate, if we added a catalyst, it would either lower the energy of the transition state or provide an alternative reaction mechanism, which might be more complex, but still has a lower overall activation energy.
Equilibrium
Importantly, catalysts don’t change the energy of the reactants or products, so they don’t impact the thermodynamic characteristics of the reaction- like the enthalpy or equilibrium constant. That is to say, when you add a catalyst, you won’t get more product, you’ll just get that product much faster. We can rephrase this to incorporate the concept of equilibrium.
Remember that most reactions reach an equilibrium with some constant amount of product and reactant, where the rate of the forward reaction is equal to the rate of the reverse reaction. When the catalyst lowers the activation energy, it lowers it for both the forward and reverse reactions. So, as we speed up the forward reaction, we’re also speeding up the reverse reaction, getting us to equilibrium faster.
Properties of Catalysts
Note that since catalysts don’t change the reactants or products, they aren’t involved in the net reaction, which means they aren’t chemically or physically changed by the process. In other words, they regenerate after each use and are free to move on and help another reactant.
Consequently, catalysts are added in nonstoichiometric amounts, meaning that you don’t need a catalyst for every mole of reactant. It’s one of those things where a little truly goes a long way. And because they’re not part of the net reaction, you’ll usually see catalysts listed above the reaction arrow in chemical equations.
Homogeneous and Heterogeneous Catalysts
Now that we’ve covered the theory of how catalysts operate, let’s explore what they look like and when they’re used.
Like I mentioned earlier, catalysts come in all shapes and sizes. For instance, synthetic catalysts are broadly split into two categories, heterogeneous and homogeneous, terms that refer to their phase. Homogeneous catalysts exist in the same phase as the reactants. In organic synthesis, acids, bases, and transition metal complexes are all frequently used to catalyze reactions in the solution phase. These are especially important in industrial processes. For example, an iridium catalyst is used in the large-scale production of acetic acid. The catalyst is in the solution with the reactants and is regenerated after each cycle.
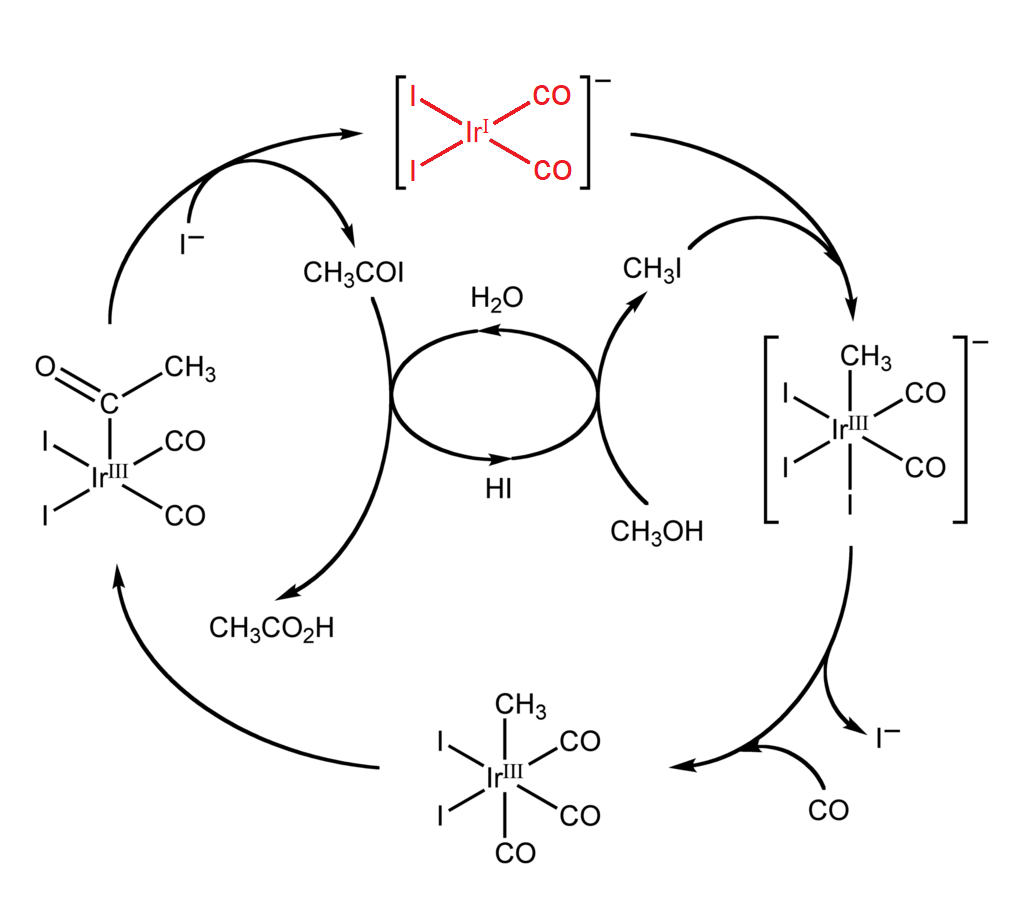
Conversely, heterogeneous catalysts exist in a separate phase from the reactants. These catalysts are often solids that catalyze reactants in the gas or liquid phase. For example, in the mass production of ammonia from nitrogen and hydrogen gases, solid iron and oxides of potassium and aluminum catalyze an otherwise very slow reaction.
This mechanism shows how the iron surface provides a location to stabilize nitrogen and hydrogen as they split into individual atoms.
As you can see, without catalysts, many of the chemicals we use on a daily basis in agriculture, pharmaceuticals, and around the house, would not be readily available!
Alright, before we go, let’s look at a quick review question:
True or false: Catalysts don’t change the energy of the reactants or products.
The correct answer is True! Catalysts can either lower the energy of the transition state or provide an alternative reaction mechanism, but they do not change the energy of the reactants or products.
I hope this review was helpful! Thanks for watching, and happy studying!